81+ pages after 1.0 mole sample of hi 800kb. What is HI at equilibrium. 075 mol c. After a 10 mole sample of HIg is placed into an evacuated 10 L container at 700. Check also: after and learn more manual guide in after 1.0 mole sample of hi If x is the equilibrium concentration of 1g the correct equilibrium constant expression is x05 - x 1 1 2x.
What is HI at equilibrium. K the reaction represented occurs.

Calculate The Enthalpy Change Of 1 Mole Of Reaction Na S 1 2 Br 2 G Rarrnabr S In Kcal Given Delta H Sub Na 137 Kj Mole 1 Deltah Bond Dissociation Br 2 G 144 Kj Mole 1 Delta H 1 St Ionisation Na G 496 Kj Mole
| Title: Calculate The Enthalpy Change Of 1 Mole Of Reaction Na S 1 2 Br 2 G Rarrnabr S In Kcal Given Delta H Sub Na 137 Kj Mole 1 Deltah Bond Dissociation Br 2 G 144 Kj Mole 1 Delta H 1 St Ionisation Na G 496 Kj Mole |
| Format: eBook |
| Number of Pages: 256 pages After 1.0 Mole Sample Of Hi |
| Publication Date: December 2020 |
| File Size: 2.1mb |
| Read Calculate The Enthalpy Change Of 1 Mole Of Reaction Na S 1 2 Br 2 G Rarrnabr S In Kcal Given Delta H Sub Na 137 Kj Mole 1 Deltah Bond Dissociation Br 2 G 144 Kj Mole 1 Delta H 1 St Ionisation Na G 496 Kj Mole |
 |
After a 10 mole sample of HIg is placed into an evacuated 10 L container at 700.

10 L container at 700K the reaction below occurs. K the reaction represented occurs. K the reaction represented above occurs. After a 10 mole sample of HIg is placed into an evacuated 10 L container at 700. A Write the expression for the equilibrium constant Kc for the reaction. K the reaction represented occurs.

A Le 9 0 5 Mole Of H2 G And 1 0 Mole Of Hi But Nol Are Added To A 1 0 L Vessel And Allowed To Reach Equilibrium According To The Following Reaction H 1
| Title: A Le 9 0 5 Mole Of H2 G And 1 0 Mole Of Hi But Nol Are Added To A 1 0 L Vessel And Allowed To Reach Equilibrium According To The Following Reaction H 1 |
| Format: eBook |
| Number of Pages: 298 pages After 1.0 Mole Sample Of Hi |
| Publication Date: April 2020 |
| File Size: 2.1mb |
| Read A Le 9 0 5 Mole Of H2 G And 1 0 Mole Of Hi But Nol Are Added To A 1 0 L Vessel And Allowed To Reach Equilibrium According To The Following Reaction H 1 |
 |
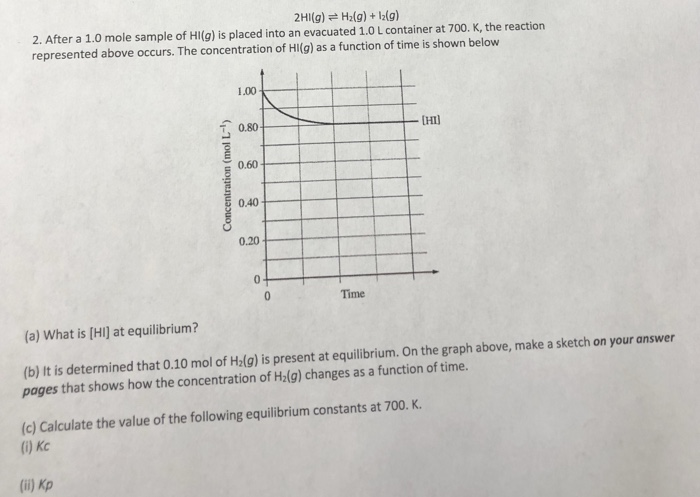
2hi G H2 G 12g 2 After A 1 0 Mole Sample Of Hl G Chegg
| Title: 2hi G H2 G 12g 2 After A 1 0 Mole Sample Of Hl G Chegg |
| Format: eBook |
| Number of Pages: 168 pages After 1.0 Mole Sample Of Hi |
| Publication Date: May 2019 |
| File Size: 1.2mb |
| Read 2hi G H2 G 12g 2 After A 1 0 Mole Sample Of Hl G Chegg |
 |

A 3 Mole Sample Of A Triatomic Ideal Gas At 300 K Is Allowed To Expand Under Adiabatic Reversible Condition From 5l To 40 L The Value Of Deltah Is
| Title: A 3 Mole Sample Of A Triatomic Ideal Gas At 300 K Is Allowed To Expand Under Adiabatic Reversible Condition From 5l To 40 L The Value Of Deltah Is |
| Format: eBook |
| Number of Pages: 192 pages After 1.0 Mole Sample Of Hi |
| Publication Date: March 2019 |
| File Size: 1.7mb |
| Read A 3 Mole Sample Of A Triatomic Ideal Gas At 300 K Is Allowed To Expand Under Adiabatic Reversible Condition From 5l To 40 L The Value Of Deltah Is |
 |

1 9701 W16 Qp 23 Moles And Stoichiometry Moles And Concentration
| Title: 1 9701 W16 Qp 23 Moles And Stoichiometry Moles And Concentration |
| Format: PDF |
| Number of Pages: 182 pages After 1.0 Mole Sample Of Hi |
| Publication Date: March 2017 |
| File Size: 1.5mb |
| Read 1 9701 W16 Qp 23 Moles And Stoichiometry Moles And Concentration |
 |

Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy
| Title: Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy |
| Format: PDF |
| Number of Pages: 296 pages After 1.0 Mole Sample Of Hi |
| Publication Date: December 2021 |
| File Size: 2.6mb |
| Read Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy |
 |

142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container
| Title: 142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container |
| Format: eBook |
| Number of Pages: 238 pages After 1.0 Mole Sample Of Hi |
| Publication Date: October 2018 |
| File Size: 1.2mb |
| Read 142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container |
 |
1 00 After A 1 0 Mole Sample Of Hi G Is Placed Into Chegg
| Title: 1 00 After A 1 0 Mole Sample Of Hi G Is Placed Into Chegg |
| Format: ePub Book |
| Number of Pages: 314 pages After 1.0 Mole Sample Of Hi |
| Publication Date: May 2021 |
| File Size: 1.9mb |
| Read 1 00 After A 1 0 Mole Sample Of Hi G Is Placed Into Chegg |
 |

At 87 C The Following Equilibrium Is Established H 2 G S S Harrh 2 S S G K P 7xx10 2 If 0 50 Mole Of Hydrogen And 1 0 Mole Of Sulphuur Are Heated To 87 C And 2 0 Atm The Equilibrium Gases Mixture
| Title: At 87 C The Following Equilibrium Is Established H 2 G S S Harrh 2 S S G K P 7xx10 2 If 0 50 Mole Of Hydrogen And 1 0 Mole Of Sulphuur Are Heated To 87 C And 2 0 Atm The Equilibrium Gases Mixture |
| Format: PDF |
| Number of Pages: 238 pages After 1.0 Mole Sample Of Hi |
| Publication Date: July 2017 |
| File Size: 2.2mb |
| Read At 87 C The Following Equilibrium Is Established H 2 G S S Harrh 2 S S G K P 7xx10 2 If 0 50 Mole Of Hydrogen And 1 0 Mole Of Sulphuur Are Heated To 87 C And 2 0 Atm The Equilibrium Gases Mixture |
 |

If 1 0 Mole Of I 2 Is Introduced In A 1 0 Litre Flask At 1000 K K C 10 6 Which One Is Correct
| Title: If 1 0 Mole Of I 2 Is Introduced In A 1 0 Litre Flask At 1000 K K C 10 6 Which One Is Correct |
| Format: ePub Book |
| Number of Pages: 129 pages After 1.0 Mole Sample Of Hi |
| Publication Date: January 2020 |
| File Size: 1.2mb |
| Read If 1 0 Mole Of I 2 Is Introduced In A 1 0 Litre Flask At 1000 K K C 10 6 Which One Is Correct |
 |

142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container Is 50 Deposed To Carbon Monoxide And Oxygen At Equilibrium 2102 Juoz 200 9 2co G O2 G
| Title: 142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container Is 50 Deposed To Carbon Monoxide And Oxygen At Equilibrium 2102 Juoz 200 9 2co G O2 G |
| Format: ePub Book |
| Number of Pages: 217 pages After 1.0 Mole Sample Of Hi |
| Publication Date: November 2020 |
| File Size: 1.8mb |
| Read 142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container Is 50 Deposed To Carbon Monoxide And Oxygen At Equilibrium 2102 Juoz 200 9 2co G O2 G |
 |
S Wongchemistry Weebly Uploads 5 1 3 6 5136424 Ap Frqs Gaseous Equilibrium Ans Pdf
| Title: S Wongchemistry Weebly Uploads 5 1 3 6 5136424 Ap Frqs Gaseous Equilibrium Ans Pdf |
| Format: PDF |
| Number of Pages: 253 pages After 1.0 Mole Sample Of Hi |
| Publication Date: April 2021 |
| File Size: 2.6mb |
| Read S Wongchemistry Weebly Uploads 5 1 3 6 5136424 Ap Frqs Gaseous Equilibrium Ans Pdf |
 |
2003B 2 HIg. After a 10 mole sample of HI g is placed into an evacuated 10 L container at 700K the reaction below occurs. The final volume of the solution is 15 L.
Here is all you need to know about after 1.0 mole sample of hi K the reaction represented above occurs. 2 HIg H2g 129 080 HI a. The concentration of HIg as a function of time is shown on the graph to the right. Calculate the enthalpy change of 1 mole of reaction na s 1 2 br 2 g rarrnabr s in kcal given delta h sub na 137 kj mole 1 deltah bond dissociation br 2 g 144 kj mole 1 delta h 1 st ionisation na g 496 kj mole if 1 0 mole of i 2 is introduced in a 1 0 litre flask at 1000 k k c 10 6 which one is correct 142 u14 at 2800 k a 1 0 mole sample of co in a one litre container is 50 deposed to carbon monoxide and oxygen at equilibrium 2102 juoz 200 9 2co g o2 g at 87 c the following equilibrium is established h 2 g s s harrh 2 s s g k p 7xx10 2 if 0 50 mole of hydrogen and 1 0 mole of sulphuur are heated to 87 c and 2 0 atm the equilibrium gases mixture a 3 mole sample of a triatomic ideal gas at 300 k is allowed to expand under adiabatic reversible condition from 5l to 40 l the value of deltah is 1 9701 w16 qp 23 moles and stoichiometry moles and concentration 20 mol -- I got.

0 Comments